Vitamin D mitigates heavy metal exposure - many studies
Vitamin D Sufficiency as a Modulator of Heavy-Metal Toxicity – Perplexity AI July 2025
A robust body of experimental, clinical, and epidemiological research indicates that maintaining serum 25-hydroxy-amin D [25(OH)D] in the generally accepted sufficiency range (50–125 nmol/L | 20–50 ng/mL) can blunt several pathophysiological effects of lead (Pb), cadmium (Cd), arsenic (As), and, to a lesser extent, mercury (Hg). Adequate vitamin D supports antioxidant defenses, metallothionein induction, calcium and phosphorus homeostasis, and immune regulation, thereby reducing metal-induced oxidative stress, nephrotoxicity, bone loss, and immunosuppression. The benefit is metal-specific, dose-dependent, and contingent on co-nutrient status (e.g., calcium, magnesium, zinc). At very high vitamin D intakes, however, risk reverses as calcium hyperabsorption and up-regulation of divalent metal transporters can facilitate Pb and Cd uptake.
Vitamin D Physiology and Metal Interactions
Vitamin D₃ (cholecalciferol) from skin or diet is hydroxylated in the liver to 25(OH)D and in renal proximal tubules to the active hormone 1,25-dihydroxy-vitamin D [1,25(OH)₂D]. Heavy metals disrupt both hydroxylation steps, whereas 1,25(OH)₂D transcriptionally up-regulates genes that can either mitigate or aggravate metal absorption and toxicity 1 2.
Dual-Edge Transporter Effect
25(OH)₂D induces divalent metal transporter-1 (DMT-1) and TRPV6, increasing gut uptake of Ca²⁺ along with Pb²⁺ and Cd²⁺ when dietary calcium is low 3 4.
Conversely, sufficiency down-regulates parathyroid hormone (PTH), stabilizes bone matrix, and reduces mobilization of Pb and Cd from skeletal stores 5 6.
Molecular Mechanisms of Protection
| Mechanistic Pathway | Protective Role of Vitamin D | Key Evidence | Notes |
| Antioxidant induction (Nrf2, SOD, CAT, GPx) | Decreases ROS and lipid peroxidation triggered by Cd and Pb 7 8 | Rat renal/testicular models, osteoblast in-vitro | Synergistic with Se, Mg, Zn |
| Metallothionein (MT) expression | Sequesters Cd²⁺, Pb²⁺, As³⁺ in non-toxic complexes 2 | Keratinocyte and renal cell cultures | MT mRNA up-regulated by 1,25(OH)₂D |
| Immunomodulation (Th17, IL-17A) | Restores T-cell proliferation suppressed by As 9 10 | Bangladesh HEALS cohort of 614 adults | Benefit observed above 20 ng/mL 25(OH)D |
| Anti-inflammatory cytokine balance | Raises IL-10, lowers TNF-α in Pb toxicity 7 | Wistar rat study | Links to reduced apoptosis |
| Mitochondrial protection | Maintains membrane potential, ATP synthesis in Cd- and Pb-exposed osteoblasts 11 | In-vitro dose-response | Requires 1–10 nM calcitriol |
| Regulation of calcium–phosphate homeostasis | Offsets Pb- and Cd-induced bone demineralization 12 13 | Human cross-sectional and animal studies | High Ca²⁺ intake essential |
Lead (Pb)
| Study Type | Population / Model | Vitamin D Status | Outcome | Direction | Citation |
| RCT (vitamin D₃ 4,000–7,000 IU/day, 12 weeks) | 44 HIV-infected youths, baseline 25(OH)D ≈ 48 nmol/L | ↑ 25(OH)D to sufficiency | No rise in blood Pb; slight inverse correlation | Protective | 26 |
| Rat study (Pb 1,000 mg/L + vitamin D 1,000 IU/kg) | Adult males, 4 weeks | Calcitriol co-admin | ↓ renal creatinine, urea; restored Ca²⁺, testosterone | Protective | 60 |
| Occupational cohort (n = 181) | Battery workers, mean BLL 38 µg/dL | Varied 25(OH)D | Higher BLL associated with lower 1,25(OH)₂D and Ca²⁺ | Detrimental deficiency | 83 |
| Pediatric longitudinal (Newark, NJ) | 142 urban children | Seasonal 25(OH)D swing | Summer 25(OH)D ↑ → blood Pb ↑1.6 µg/dL | Facilitative when Ca²⁺ low | 22 |
Interpretation: Adequate vitamin D with concurrent calcium sufficiency appears renoprotective and anti-inflammatory, whereas seasonal spikes without mineral support can enhance Pb absorption.
Cadmium (Cd)
| Study | Design | Key Finding | Citation |
| Chinese community, n = 133 | Quartile analysis | 25(OH)D ≥ 40 ng/mL cut Cd-induced tubular dysfunction risk by 80% | 2 |
| School-age Polish children, n = 140 | High vs. low Cd blood | High Cd group had 23% lower 25(OH)D; inverse correlation with oxidative markers | 6 |
| Rat nephropathy model | Cd 3 mg/kg, vitamin D + Ca co-therapy | Normalized CaSR, decreased ROS, improved histology | 10 |
| In-vitro osteoblasts | Cd/Pb 1–10 µM with 1–10 nM calcitriol | Calcitriol restored mitochondrial function, ↓ apoptosis | 59 |
Interpretation: Vitamin D sufficiency markedly mitigates Cd renal and skeletal toxicity via MT induction and antioxidant reinforcement, provided Ca²⁺/Mg²⁺ intake is adequate.
Arsenic (As)
| Evidence | Population | Vitamin D Threshold | Protective Endpoint | Citation |
| HEALS cohort, Bangladesh | 614 adults | >20 ng/mL | Prevented As-related suppression of T-cell proliferation | 84 |
| U.S. pregnant women (BKMR) | 1,573 | Higher 25(OH)D linked to lower deficiency prevalence despite As | Vitamin D moderates VDD risk | 14 |
| Mouse keratinocytes | Calcitriol pre-treatment | Inhibited As uptake (AQP7/9/10 down-regulation), ↓ MEK/ERK signaling | Anti–tumorigenic | 3 |
| Therapeutic synergy | Paricalcitol + arsenic trioxide in leukemia lines | Enhanced apoptosis, ↓ PML-RARA oncoprotein | Beneficial in malignancy | 3 |
Interpretation: Vitamin D sufficiency (>20 ng/mL) confers immune and epithelial protection against arsenic; pharmacological VDR agonists may enhance arsenic-based chemotherapies.
Mercury (Hg)
| Model / Study | Outcome of Adequate Vitamin D | Limitation | Citation |
| Dolphin keratinocytes, MeHg | MeHg suppressed VDR signaling; vitamin D deficiency worsened effect | Cell-specific | 24 |
| Korean postmenopausal women, n = 1,134 | Positive Hg-25(OH)D correlation driven by fish intake, confounded | Diet confounding | 49 |
| Rat HgCl₂ toxicity with vitamin E/Zn | Combination (no vitamin D) showed organ protection; analogous antioxidant role for D hypothesized | Indirect | 69 |
| Review of MeHg toxicity | Vitamin D may bolster glutathione and metallothionein, but data sparse | Need trials | 42 |
Interpretation: Direct protective evidence is limited; however, mechanistic data suggest potential benefits through antioxidant and MT pathways. Dietary co-exposure complicates epidemiology.
Summary Table of Metals
| Heavy Metal | Primary Target Organs | Does Vitamin D Sufficiency Mitigate Toxicity? | Optimal 25(OH)D Range Suggested by Data | Key Caveat |
| Lead (Pb) | Kidney, bone, CNS | Yes—reduces renal oxidative stress and testicular injury 7 14 | 50–100 nmol/L; must pair with Ca²⁺ | High 25(OH)D with low Ca²⁺ ↑ Pb uptake 15 |
| Cadmium (Cd) | Kidney, bone, lung | Strong—cuts tubular dysfunction, boosts MT 16 17 | ≥100 nmol/L with Ca/Mg/Zn adequacy | Excess D without minerals ↑ Cd absorption 2 |
| Arsenic (As) | Immune, skin, lung | Moderate—protects T-cell proliferation, inhibits skin oncogenesis 9 18 | >50 nmol/L | Data sparse above 125 nmol/L |
| Mercury (Hg) | CNS, kidney | Inconclusive—cell models suggest benefit; human data confounded 19 20 | Unknown | Dietary fish confounds; more trials needed |
Screening and Supplementation implications
Populations at Risk: Industrial workers (Pb, Cd), residents of As-contaminated aquifers, smokers, pregnant women, and children require dual monitoring of heavy metals and 25(OH)D 21 22.
Target Range: Maintain 25(OH)D between 75–100 nmol/L (30–40 ng/mL) while ensuring calcium 1,000–1,200 mg/day and magnesium 300–400 mg/day to prevent transporter-mediated metal uptake 4 23.
Dosing: 1,000–2,000 IU/day vitamin D₃ suffices for most adults; avoid chronic intakes ≥10,000 IU/day unless medically supervised, as hypercalcemia can precipitate nephrocalcinosis and AKI 24 25.
Therapeutic Potential implications
Adjunct in Chelation: Calcitriol or paricalcitol may enhance MT expression, aiding chelation of Pb and Cd; clinical trials in chronic kidney disease are warranted 7 26.
Onco-Immunology: Combination of vitamin D analogs with arsenic trioxide shows promise in leukemia treatment through synergistic apoptosis 18.
Nutritional Synergy implications
Combine vitamin D with adequate Ca, Mg, Zn, Se to reinforce protective metallothionein networks and antioxidant systems 2 27.
Emphasize low-trophic fish (sardines) to obtain vitamin D without high MeHg burden 28.
Research Gaps and Future Directions
Prospective Cohorts: Serial bone Pb, urinary Cd, hair Hg, and 25(OH)D trajectories to delineate causality.
Dose-Response Trials: Determine vitamin D thresholds where protection shifts to facilitation of metal uptake.
Gene–Environment Interactions: Explore VDR and MT polymorphisms in metal susceptibility 26 29.
Mercury-Specific Studies: Randomized supplementation in high-fish consumers to assess neurocognitive outcomes.
Mixed-Metal Models: Apply Bayesian kernel machine regression to parse interactive effects of Pb–Cd–As under varying vitamin D status 30.
Conclusion
A “good” vitamin D status—defined as serum 25(OH)D in the lower-to-mid sufficiency range—generally reduces the toxicity of lead, cadmium, and arsenic , chiefly by bolstering antioxidant defenses, inducing metallothioneins, and stabilizing calcium metabolism. Evidence for mercury is less definitive but biologically plausible. The protective window narrows when vitamin D intakes become excessive or when mineral cofactors are lacking, conditions that can paradoxically heighten Pb and Cd absorption. Integrative strategies that optimize vitamin D and essential minerals while minimizing metal exposure offer a tangible route to mitigating heavy-metal health risks.
References
https://www.sciencedirect.com/science/article/pii/S2773050623000502
https://academic.oup.com/nutritionreviews/article-pdf/39/10/372/24082074/nutritionreviews39-0372.pdf
https://ec.bioscientifica.com/view/journals/ec/10/4/EC-21-0006.xml
https://ui.adsabs.harvard.edu/abs/2023JTEM....600097A/abstract
https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0266168
https://www.healthline.com/health-news/too-much-vitamin-d-can-lead-to-kidney-failure
https://www.sciencedirect.com/science/article/abs/pii/S0946672X23000974
https://www.grassrootshealth.net/blog/glutathione-vitamin-d-relationship/
https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2024.1371920/full
https://www.linkedin.com/pulse/can-cadmium-cause-your-vitamin-d-deficiency-upgradedformulas-tgeqe
https://www.sciencedirect.com/science/article/abs/pii/S0013935124022734
https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0234965
https://archive.cdc.gov/wwwatsdrcdc_gov/csem/cadmium/Chronic-Effects.html
https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0195682
https://publishing.emanresearch.org/Journal/Abstract/angiotherapy-829453
https://www.sciencedirect.com/science/article/abs/pii/S0165032724012175
https://onlinelibrary.wiley.com/doi/10.1097/MPG.0b013e3182758c4a
https://www.sciencedirect.com/science/article/abs/pii/S1382668919301176
https://archive.cdc.gov/wwwatsdrcdcgov/csem/leadtoxicity/physiologicaleffects.html
https://www.scitechnol.com/peer-review/vitamin-d-geneticsenvironmenthealth-z9VQ.php?article_id=2347
https://www.sciencedirect.com/science/article/pii/S0013935122003619
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2018.00477/full
https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2018.00550/full
https://publications.aap.org/pediatrics/article/87/5/680/56971/Serum-Vitamin-D-Metabolites-and-Bone
https://www.sciencedirect.com/science/article/pii/S0022316623019818
https://www.sciencedirect.com/science/article/pii/000629529190617E
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2013.00148/full
https://www.frontiersin.org/journals/molecular-neuroscience/articles/10.3389/fnmol.2018.00125/full
https://www.degruyterbrill.com/document/doi/10.1515/bc.2010.014/html?lang=en
https://mothertobaby.org/fact-sheets/methylmercury-pregnancy/
https://www.sciencedirect.com/science/article/abs/pii/S0013935115301857
https://www.sciencedirect.com/science/article/pii/S109727650080413X
https://www.sciencedirect.com/science/article/abs/pii/S1382668909001689
https://www.grassrootshealth.net/blog/master-antioxidant-glutathione-affected-vitamin-d-status/
https://anatomypubs.onlinelibrary.wiley.com/doi/10.1002/ar.22485
https://www.epa.gov/sites/default/files/2015-09/documents/volume5.pdf
https://journals.sagepub.com/doi/full/10.1177/0960327116677355
https://www.sciencedirect.com/science/article/abs/pii/S0960076011001506
https://www.sciencedirect.com/science/article/pii/S0022316623020345
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/calcitriol
https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI%3A17823
1. Vitamin D mitigates Lead, Cadmium, and Arsenic, but not Mercury - Perplexity AI July 2025 1. Impact of Heavy Metals on the Antioxidant Activity of Vitamin D: A Metabolic Perspective - July 2025Metabolites 2025, 15(7), 440; https://doi.org/10.3390/metabo15070440
by Ji Seo Park 1,2,†ORCID,Mi-Ri Gwon 1,2,3,†,Jae Hwa Lee 1,2ORCID,Jin Ju Park 1,2,Hae Won Lee 1,2,Duk-Hee Lee 4ORCID,Sook Jin Seong 1,2,3,* andYoung-Ran Yoon 1,2,3,*
Background/Objectives: Vitamin D (VD) is metabolized in the body and plays a crucial role in regulating the antioxidant system. While exposure to heavy metals (HMs) inhibits VD activity, HMs can also be absorbed following VD stimulation. Despite differing views on the interaction between HM and VD activity, the effects of HM exposure on VD-related pathways have not been examined using metabolomics. This study aimed to investigate the impact of HM exposure on VD-related antioxidant activity under VD deficiency conditions using untargeted metabolic profiling.
Methods: In this retrospective cohort study, 46 plasma samples were analyzed using ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC-QTOF/MS). Metabolic profiling was performed on two groups: individuals with severe VD deficiency and low HM exposure (SVDD–LHM) and those with VD deficiency and high HM exposure (VDD–HHM).
Results: As a compensatory response to oxidative stress induced by HMs, VD-related antioxidant pathways may be associated with elevated levels of antioxidants, including bilirubin, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). In-creases in EPA and DHA were also linked to alterations in lipid metabolism, including diacylglycerol and phosphatidylcholine levels. DHA showed an area under the curve (AUC) of 0.850 (95% CI: 0.651–0.990), suggesting that DHA could serve as a potential biomarker for VD activity in response to HM exposure.
Conclusions: The identified metabolites and metabolic pathways suggest that HM exposure may stimulate VD-related antioxidant activity, even under VD-deficient conditions.
📄 Download the PDF from Vitamin D Life
1. Vitamin D Levels and Heavy Metals Exposure in Pregnancy and Childbirth - Nov 2024Interplay Between Vitamin D Levels and Heavy Metals Exposure in Pregnancy and Childbirth: A Systematic Review
by Tania Flores-Bazán 1ORCID,Jeannett Alejandra Izquierdo-Vega 2ORCID,José Antonio Guerrero-Solano 3ORCID,Araceli Castañeda-Ovando 4ORCID,Diego Estrada-Luna 1ORCID andAngélica Saraí Jiménez-Osorio 1,*ORCID MEXICO
Background/Objectives: Vitamin D (VD) deficiency has been associated with increased risk of gestational disorders affecting the endocrine system, immune system, and neurodevelopment in offspring. Recent studies have focused on the interaction between toxic elements and micronutrients during pregnancy. This review analyzes the potential relationships between VD levels and heavy metals in pregnant women and their offspring. Methods: A systematic review was conducted according to PRISMA 2020 guidelines, using databases such as PubMed, ScienceDirect, Cochrane Library, and Google Scholar. Boolean operators ‘AND’ and ‘OR’ were applied with terms like ‘pregnancy’, ‘vitamin D’, ‘heavy metals’, and ‘newborns’.
Results: From 4688 articles, 14 studies were selected based on relevance and quality. These studies measured the levels of metals like lead (Pb), cadmium (Cd), mercury (Hg), and arsenic (As), in biological samples including maternal blood, umbilical cord blood, placenta tissue, and meconium during different stages of pregnancy, showing an inverse relationship between VD deficiency and heavy metal concentrations, which could be related to the incidence of preterm birth.
Conclusions: The review highlights the importance of maintaining adequate VD levels during pregnancy, suggesting that sufficient VD may mitigate the adverse effects of heavy metal exposure, potentially reducing pregnancy-related complications.
📄 Download the PDF from Vitamin D Life
References
Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96,1911-1930.
Poel, Y.; Hummel, P.; Lips, P.; Stam, F.; Van Der Ploeg, T.; Simsek, S. Vitamin D and gestational diabetes: A systematic review and meta-analysis. Eur. J. Intern. Med. 2012,23, 465-469.
Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O'Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169.
Zhang, M.-X.; Pan, G.-T.; Guo, J.-F.; Li, B.-Y.; Qin, L.-Q.; Zhang, Z.-L. Vitamin D deficiency increases the risk of gestational diabetes mellitus: A meta-analysis of observational studies. Nutrients 2015, 7, 8366-8375.
Tabesh, M.; Salehi-Abargouei, A.; Tabesh, M.; Esmaillzadeh, A. Maternal vitamin D status and risk of pre-eclampsia: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 3165-3173.
Akbari, S.; Khodadadi, B.; Ahmadi, S.A.Y.; Abbaszadeh, S.; Shahsavar, F. Association of vitamin D level and vitamin D deficiency with risk of preeclampsia: A systematic review and updated meta-analysis. Taiwan. J. Obstet. Gynecol. 2018, 57, 241-247.
Yuan, Y.; Tai, W.; Xu, P.; Fu, Z.; Wang, X.; Long, W.; Guo, X.; Ji, C.; Zhang, L.; Zhang, Y. Association of maternal serum 25-hydroxyvitamin D concentrations with risk of preeclampsia: A nested case-control study and meta-analysis. J. Matern. Fetal Neonatal Med. 2021, 34,1576-1585.
Lima, M.S.; Pereira, M.; Castro, C.T.; Santos, D.B. Vitamin D deficiency and anemia in pregnant women: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 428-438.
Qin, L.-L.; Lu, F.-G.; Yang, S.-H.; Xu, H.-L.; Luo, B.-A. Does maternal vitamin D deficiency increase the risk of preterm birth: A meta-analysis of observational studies. Nutrients 2016, 8, 301.
Lian, R.-H.; Qi, P.-A.; Yuan, T.; Yan, P.-J.; Qiu, W.-W.; Wei, Y.; Hu, Y.-G.; Yang, K.-H.; Yi, B. Systematic review and meta-analysis of vitamin D deficiency in different pregnancy on preterm birth: Deficiency in middle pregnancy might be at risk. Medicine 2021, 100, e26303.
Zhang, H.; Huang, Z.; Xiao, L.; Jiang, X.; Chen, D.; Wei, Y. Meta-analysis of the effect of the maternal vitamin D level on the risk of spontaneous pregnancy loss. Int. J. Gynecol. Obstet. 2017,138, 242-249.
Tamblyn, J.A.; Pilarski, N.S.; Markland, A.D.; Marson, E.J.; Devall, A.; Hewison, M.; Morris, R.K.; Coomarasamy, A. Vitamin D and miscarriage: A systematic review and meta-analysis. Fertil. Steril. 2022,118,111-122.
Chen, C.; Wang, S.; Zhang, C.; Wu, X.; Zhou, L.; Zou, X.; Guan, T.; Zhang, Z.; Hao, J. Association between serum vitamin D level during pregnancy and recurrent spontaneous abortion: A systematic review and meta-analysis. Am. J. Reprod. Immunol. 2022, 88, e13582.
Szarpak, L.; Feduniw, S.; Pruc, M.; Ciebiera, M.; Cander, B.; Rahnama-Hezavah, M.; Szarpak, L. The Vitamin D Serum Levels in Pregnant Women Affected by COVID-19: A Systematic Review and Meta-Analysis. Nutrients 2023,15, 2588.
Chen, Y.; Zhu, B.; Wu, X.; Li, S.; Tao, F. Association between maternal vitamin D deficiency and small for gestational age: Evidence from a meta-analysis of prospective cohort studies. BMJ Open 2017, 7, e016404.
Thacher, T.D.; Clarke, B.L. Vitamin D insufficiency. Mayo Clin. Proc. 2011, 86, 50-60.
Tsiaras, W.G.; Weinstock, M.A. Factors influencing vitamin D status. Acta Derm. Venereol. 2011, 91,115.
Vidailhet, M.; Mallet, E.; Bocquet, A.; Bresson, J.-L.; Briend, A.; Chouraqui, J.-P.; Darmaun, D.; Dupont, C.; Frelut, M.-L.; Ghisolfi, J. Vitamin D: Still a topical matter in children and adolescents. A position paper by the Committee on Nutrition of the French Society of Paediatrics. Arch. Pediatr. 2012,19, 316-328.
Chee, W.F.; Aji, A.S.; Lipoeto, N.I.; Siew, C.Y. Maternal vitamin D status and its associated environmental factors: A cross-sectional study. Ethiop. J. Health Sci. 2022, 32, 885-894.
Zhao, Y.; Wang, L.; Liu, H.; Cao, Z.; Su, X.; Cai, J.; Hua, J. Particulate air pollution exposure and plasma vitamin D levels in pregnant women: A longitudinal cohort study. J. Clin. Endocrinol. Metab. 2019,104, 3320-3326.
Yang, D.; Chen, L.; Yang, Y.; Shi, J.; Huang, Z.; Li, M.; Yang, Y.; Ji, X. Effect of PM2. 5 exposure on Vitamin D status among pregnant women: A distributed lag analysis. Ecotoxicol. Environ. Saf. 2022, 239,113642.
Baiz, N.; Dargent-Molina, P.; Wark, J.D.; Souberbielle, J.-C.; Slama, R.; Annesi-Maesano, I.; Group, E.M.-C.C.S. Gestational exposure to urban air pollution related to a decrease in cord blood vitamin D levels. J. Clin. Endocrinol. Metab. 2012, 97,4087-4095.
Johns, L.E.; Ferguson, K.K.; Cantonwine, D.E.; McElrath, T.F.; Mukherjee, B.; Meeker, J.D. Erratum:“Urinary BPA and Phthalate Metabolite Concentrations and Plasma Vitamin D Levels in Pregnant Women: A Repeated Measures Analysis". Environ. Health Perspect. 2019,127, 019002.
Mousavi, S.E.; Amini, H.; Heydarpour, P.; Chermahini, F.A.; Godderis, L. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms. Environ. Int. 2019,122, 67-90.
Long, J.; Huang, H.; Tang, P.; Liang, J.; Liao, Q.; Chen, J.; Pang, L.; Yang, K.; Wei, H.; Chen, M. Associations between maternal exposure to multiple metals and metalloids and blood pressure in preschool children: A mixture-based approach. J. Trace Elem. Med. Biol. 2024, 84,127460.
Xu, R.; Meng, X.; Pang, Y.; An, H.; Wang, B.; Zhang, L.; Ye, R.; Ren, A.; Li, Z.; Gong, J. Associations of maternal exposure to 41 metals/metalloids during early pregnancy with the risk of spontaneous preterm birth: Does oxidative stress or DNA methylation play a crucial role? Environ. Int. 2022,158,106966.
Caserta, D.; Graziano, A.; Monte, G.; Bordi, G.; Moscarini, M. heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur. Rev. Med. Pharmacol. Sci. 2013,17, 2198-2206.
Bauer, J.A.; Romano, M.E.; Jackson, B.P.; Bellinger, D.; Korrick, S.; Karagas, M.R. Associations of Perinatal Metal and Metalloid Exposures with Early Child Behavioral Development Over Time in the New Hampshire Birth Cohort Study. Expo. Health 2024, 16,135-148.
Mitra, P.; Sharma, S.; Purohit, P.; Sharma, P. Clinical and molecular aspects of lead toxicity: An update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 506-528.
Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71.
Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Systematic Reviews of Etiology and Risk; Aromataris, E., Lockwood, C., Porritt, K., Pilla, B., Jordan, Z., Eds.; JBI Manual for Evidence Synthesis; JBI: Adelaide, SA, Australia, 2020.
Zhang, J.; Bai, Y.; Chen, X.; Li, S.; Meng, X.; Jia, A.; Yang, X.; Huang, F.; Zhang, X.; Zhang, Q. Association between urinary arsenic species and vitamin D deficiency: A cross-sectional study in Chinese pregnant women. Front. Public Health 2024,12,1371920.
Fisher, M.; Marro, L.; Arbuckle, T.E.; Potter, B.K.; Little, J.; Weiler, H.; Morisset, A.S.; Lanphear, B.; Oulhote, Y.; Braun, J.M. Association between toxic metals, vitamin D and preterm birth in the Maternal-Infant research on environmental chemicals study. Paediatr. Perinat. Epidemiol. 2023, 37, 447-457.
Fisher, M.; Potter, B.; Little, J.; Oulhote, Y.; Weiler, H.A.; Fraser, W.; Morisset, A.-S.; Braun, J.; Ashley-Martin, J.; Borghese, M.M. Blood metals and vitamin D status in a pregnancy cohort: A bidirectional biomarker analysis. Environ. Res. 2022, 211,113034.
Jukic, A.M.Z.; Kim, S.S.; Meeker, J.D.; Weiss, S.T.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. A prospective study of maternal 25-hydroxyvitamin D (25OHD) in the first trimester of pregnancy and second trimester heavy metallevels. Environ. Res. 2021,199,111351.
Fang, X.; Qu, J.; Huan, S.; Sun, X.; Li, J.; Liu, Q.; Jin, S.; Xia, W.; Xu, S.; Wu, Y. Associations of urine metals and metal mixtures during pregnancy with cord serum vitamin D Levels: A prospective cohort study with repeated measurements of maternal urinary metal concentrations. Environ. Int. 2021,155,106660.
Wu, H.; Xu, B.; Guan, Y.; Chen, T.; Huang, R.; Zhang, T.; Sun, R.; Xie, K.; Chen, M. A metabolomic study on the association of exposure to heavy metals in the first trimester with primary tooth eruption. Sci. Total Environ. 2020, 723,138107.
Jukic, A.M.Z.; Zuchniak, A.; Qamar, H.; Ahmed, T.; Mahmud, A.A.; Roth, D.E. Vitamin d treatment during pregnancy and maternal and neonatal cord blood metal concentrations at delivery: Results of a randomized controlled trial in Bangladesh. Environ. Health Perspect. 2020,128,117007.
Irwinda, R.; Wibowo, N.; Putri, A.S. The concentration of micronutrients and heavy metals in maternal serum, placenta, and cord blood: A cross-sectional study in preterm birth. J. Pregnancy 2019,2019, 5062365.
Kucukaydin, Z.; Kurdoglu, M.; Kurdoglu, Z.; Demir, H.; Yoruk, I.H. Selected maternal, fetal and placental trace element and heavy metaland maternal vitamin levels in preterm deliveries with or without preterm premature rupture of membranes. J. Obstet. Gynaecol. Res. 2018, 44, 880-889.
Harari, F.; Akesson, A.; Casimiro, E.; Lu, Y.; Vahter, M. Exposure to lithium through drinking water and calcium homeostasis during pregnancy: A longitudinal study. Environ. Res. 2016,147,1-7.
Arbuckle, T.E.; Liang, C.L.; Morisset, A.-S.; Fisher, M.; Weiler, H.; Cirtiu, C.M.; Legrand, M.; Davis, K.; Ettinger, A.S.; Fraser, W.D. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 2016, 163, 270-282.
Rezende, V.B.; Amaral, J.H.; Quintana, S.M.; Gerlach, R.F.; Barbosa, F., Jr.; Tanus-Santos, J.E. Vitamin D receptor haplotypes affect lead levels during pregnancy. Sci. Total Environ. 2010, 408, 4955-4960.
Kolusari, A.; Adali, E.; Kurdoglu, M.; Yildizhan, R.; Cebi, A.; Edirne, T.; Demir, H.; Yoruk, I.H. Catalase activity, serum trace element and heavy metalconcentrations, vitamin A, vitamin D and vitamin E levels in hydatidiform mole. Clin. Exp. Obstet. Gynecol. 2009, 36,102-104.
Kolusari, A.; Kurdoglu, M.; Yildizhan, R.; Adali, E.; Edirne, T.; Cebi, A.; Demir, H.; Yoruk, I.H. Catalase activity, serum trace element and heavy metalconcentrations, and vitamin A, D and E levels in pre-eclampsia. J. Int. Med. Res. 2008, 36,1335-1341.
Salle, B.L.; Delvin, E.E.; Lapillonne, A.; Bishop, N.J.; Glorieux, F.H. Perinatal metabolism of vitamin D. Am. J. Clin. Nutr. 2000, 71,1317s-1324s.
Liu, Y.; Ding, C.; Xu, R.; Wang, K.; Zhang, D.; Pang, W.; Tu, W.; Chen, Y. Effects of vitamin D supplementation during pregnancy on offspring health at birth: A meta-analysis of randomized controlled trails. Clin. Nutr. 2022, 41,1532-1540.
Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2013, 71,118-132.
Forsby, M.; Winkvist, A.; Barebring, L.; Augustin, H. Supplement use in relation to dietary intake in pregnancy: An analysis of the Swedish GraviD cohort. Br. J. Nutr. 2024,131, 256-264.
Holick, M.F. Vitamin D: Evolutionary, physiological and health perspectives. Curr. Drug Targets 2011,12, 4-18.
Kinney, D.K.; Teixeira, P.; Hsu, D.; Napoleon, S.C.; Crowley, D.J.; Miller, A.; Hyman, W.; Huang, E. Relation of schizophrenia prevalence to latitude, climate, fish consumption, infant mortality, and skin color: A role for prenatal vitamin d deficiency and infections? Schizophr. Bull. 2009, 35, 582-595.
Alp, H.; Tekgunduz, K.; Akkar, M.K. Maternal and cord blood vitamin D status in high-altitude pregnancy. J. Matern. Fetal Neonatal Med. 2016, 29, 571-575.
Vergara-Maldonado, C.; Urdaneta-Machado, J.R. The Effects of Latitude and Temperate Weather on Vitamin D Deficiency and Women's Reproductive Health: A Scoping Review. J. Midwifery Womens Health 2023, 68, 340-352.
Merewood, A.; Mehta, S.D.; Grossman, X.; Chen, T.C.; Mathieu, J.S.; Holick, M.F.; Bauchner, H. Widespread vitamin D deficiency in urban Massachusetts newborns and their mothers. Pediatrics 2010,125, 640-647.
Li, W.; Green, T.J.; Innis, S.M.; Barr, S.I.; Whiting, S.J.; Shand, A.; von Dadelszen, P. Suboptimal vitamin D levels in pregnant women despite supplement use. Can. J. Public Health 2011,102, 308-312.
Tian, Y.; Holzman, C.; Siega-Riz, A.M.; Williams, M.A.; Dole, N.; Enquobahrie, D.A.; Ferre, C.D. Maternal Serum 25-Hydroxyvitamin D Concentrations during Pregnancy and Infant Birthweight for Gestational Age: A Three-Cohort Study. Paediatr. Perinat. Epidemiol. 2016, 30,124-133.
Wegienka, G.; Kaur, H.; Sangha, R.; Cassidy-Bushrow, A.E. Maternal-Cord Blood Vitamin D Correlations Vary by Maternal Levels. J. Pregnancy 2016,2016, 7474192.
Alanazi, M.; Nabil Aboushady, R.M.; Kamel, A.D. Association between different levels of maternal vitamin-D status during pregnancy and maternal outcomes. Clin. Nutr. ESPEN 2022, 50, 307-313.
Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014,21, 319-329.
Tsuprykov, O.; Buse, C.; Skoblo, R.; Hocher, B. Comparison of free and total 25-hydroxyvitamin D in normal human pregnancy. J. Steroid Biochem. Mol. Biol. 2019,190, 29-36.
Ramasamy, I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020, 41,103-126.
Leffelaar, E.R.; Vrijkotte, T.G.; van Eijsden, M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: Results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br. J. Nutr. 2010,104,108-117.
Judistiani, R.T.D.; Nirmala, S.A.; Rahmawati, M.; Ghrahani, R.; Natalia, Y.A.; Sugianli, A.K.; Indrati, A.R.; Suwarsa, O.; Setiabudiawan, B. Optimizing ultraviolet B radiation exposure to prevent vitamin D deficiency among pregnant women in the tropical zone: Report from cohort study on vitamin D status and its impact during pregnancy in Indonesia. BMC Pregnancy Childbirth 2019,19, 209.
Yun, C.; Chen, J.; He, Y.; Mao, D.; Wang, R.; Zhang, Y.; Yang, C.; Piao, J.; Yang, X. Vitamin D deficiency prevalence and risk factors among pregnant Chinese women. Public Health Nutr. 2017, 20,1746-1754.
Gulson, B.; Mizon, K.; Korsch, M.; Taylor, A. Revisiting mobilisation of skeletal lead during pregnancy based on monthly sampling and cord/maternal blood lead relationships confirm placental transfer of lead. Arch. Toxicol. 2016, 90, 805-816.
Tasin, F.R.; Ahmed, A.; Halder, D.; Mandal, C. On-going consequences of in utero exposure of Pb: An epigenetic perspective. J. Appl. Toxicol. 2022, 42,1553-1569.
Tung, P.W.; Kennedy, E.M.; Burt, A.; Hermetz, K.; Karagas, M.; Marsit, C.J. Prenatal lead (Pb) exposure is associated with differential placental DNA methylation and hydroxymethylation in a human population. Epigenetics 2022, 17, 2404-2420.
RÍsovÁ, V. The pathway of lead through the mother's body to the child. Interdiscip. Toxicol. 2019,12,1-6.
Taylor, C.M.; Doerner, R.; Northstone, K.; Kordas, K. Maternal Diet During Pregnancy and Blood Cadmium Concentrations in an Observational Cohort of British Women. Nutrients 2020,12, 904.
Kovacs, G.; Danko, T.; Bergeron, M.J.; Balazs, B.; Suzuki, Y.; Zsembery, A.; Hediger, M.A. heavy metal** cations permeate the TRPV6 epithelial cation channel. Cell Calcium 2011, 49, 43-55.
Nakamura, Y.; Ohba, K.; Ohta, H. Participation of metal transporters in cadmium transport from mother rat to fetus. J. Toxicol. Sci. 2012, 37, 1035-1044.
Geng, H.X.; Wang, L. Cadmium: Toxic effects on placental and embryonic development. Environ. Toxicol. Pharmacol. 2019, 67,102-107.
Castillo, P.; Ibáñez, F.; Guajardo, A.; Llanos, M.N.; Ronco, A.M. Impact of cadmium exposure during pregnancy on hepatic glucocorticoid receptor methylation and expression in rat fetus. PLoS ONE 2012, 7, e44139.
Jacobo-Estrada, T.; Santoyo-Sánchez, M.; Thévenod, F.; Barbier, O. Cadmium Handling, Toxicity and Molecular Targets Involved during Pregnancy: Lessons from Experimental Models. Int. J. Mol. Sci. 2017,18,1590.
Kippler, M.; Tofail, F.; Gardner, R.; Rahman, A.; Hamadani, J.D.; Bottai, M.; Vahter, M. Maternal cadmium exposure during pregnancy and size at birth: A prospective cohort study. Environ. Health Perspect. 2012,120, 284-289.
Bose-O'Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury exposure and children's health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40,186-215.
Hoffmeyer, R.E.; Singh, S.P.; Doonan, C.J.; Ross, A.R.; Hughes, R.J.; Pickering, I.J.; George, G.N. Molecular mimicry in mercury toxicology. Chem. Res. Toxicol. 2006,19, 753-759.
Simmons-Willis, T.A.; Koh, A.S.; Clarkson, T.W.; Ballatori, N. Transport of a neurotoxicant by molecular mimicry: The methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem. J. 2002, 367, 239-246.
Straka, E.; Ellinger, I.; Balthasar, C.; Scheinast, M.; Schatz, J.; Szattler, T.; Bleichert, S.; Saleh, L.; Knófler, M.; Zeisler, H.; et al. Mercury toxicokinetics of the healthy human term placenta involve amino acid transporters and ABC transporters. Toxicology 2016, 340, 34-42.
Tong, M.; Yu, J.; Liu, M.; Li, Z.; Wang, L.; Yin, C.; Ren, A.; Chen, L.; Jin, L. Total mercury concentration in placental tissue, a good biomarker of prenatal mercury exposure, is associated with risk for neural tube defects in offspring. Environ. Int. 2021, 150,106425.
Ealo Tapia, D.; Torres Abad, J.; Madera, M.; Márquez Lázaro, J. Mercury and neurodevelopmental disorders in children: A systematic review. Arch. Argent. Pediatr. 2023,121, e202202838.
Cubadda, F.; D'Amato, M.; Mancini, F.R.; Aureli, F.; Raggi, A.; Busani, L.; Mantovani, A. Assessing human exposure to inorganic arsenic in high-arsenic areas of Latium: A biomonitoring study integrated with indicators of dietary intake. Ann. Ig. 2015, 27, 39-51.
Rehman, K.; Naranmandura, H. Arsenic metabolism and thioarsenicals. Metallomics 2012, 4, 881-892.
El-Ghiaty, M.A.; El-Kadi, A.O.S. The Duality of Arsenic Metabolism: Impact on Human Health. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 341-358.
Agency for Toxic Substances and Disease Registry. Table 8-1 Regulations and Guidelines Applicable to Arsenic and Arsenic Compounds. Toxicological Profile for Arsenic. 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK591644/table/ ch8.tab1/ (accessed on 10 September 2024).
Lewis, J.V.; Knapp, E.A.; Bakre, S.; Dickerson, A.S.; Bastain, T.M.; Bendixsen, C.; Bennett, D.H.; Camargo, C.A.; Cassidy-Bushrow, A.E.; Colicino, E.; et al. Associations between area-level arsenic exposure and adverse birth outcomes: An Echo-wide cohort analysis. Environ. Res. 2023, 236,116772.
Shi, X.; Ayotte, J.D.; Onda, A.; Miller, S.; Rees, J.; Gilbert-Diamond, D.; Onega, T.; Gui, J.; Karagas, M.; Moeschler, J. Geospatial association between adverse birth outcomes and arsenic in groundwater in New Hampshire, USA. Environ. Geochem. Health 2015, 37,333-351.
Quansah, R.; Armah, F.A.; Essumang, D.K.; Luginaah, I.; Clarke, E.; Marfoh, K.; Cobbina, S.J.; Nketiah-Amponsah, E.; Namujju, P.B.; Obiri, S.; et al. Association of arsenic with adverse pregnancy outcomes/infant mortality: A systematic review and meta-analysis. Environ. Health Perspect. 2015,123,412-421.
Salmeri, N.; Villanacci, R.; Ottolina, J.; Bartiromo, L.; Cavoretto, P.; Dolci, C.; Lembo, R.; Schimberni, M.; Valsecchi, L.; Vigano, P.; et al. Maternal Arsenic Exposure and Gestational Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2020,12, 3094.
Stone, J.; Sutrave, P.; Gascoigne, E.; Givens, M.B.; Fry, R.C.; Manuck, T.A. Exposure to toxic metals and per- and polyfluoroalkyl substances and the risk of preeclampsia and preterm birth in the United States: A review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100308.
Ashley-Martin, J.; Fisher, M.; Belanger, P.; Cirtiu, C.M.; Arbuckle, T.E. Biomonitoring of inorganic arsenic species in pregnancy. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 921-932.
Edelstein, S.; Fullmer, C.S.; Wasserman, R.H. Gastrointestinal absorption of lead in chicks: Involvement of the cholecalciferol endocrine system. J. Nutr. 1984,114, 692-700.
Ngueta, G.; Gonthier, C.; Levallois, P. Colder-to-warmer changes in children's blood lead concentrations are related to previous blood lead status: Results from a systematic review of prospective studies. J. Trace Elem. Med. Biol. 2015, 29, 39-46.
Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019,186, 4-21.
Tissandie, E.; Gueguen, Y.; Lobaccaro, J.M.; Grandcolas, L.; Grison, S.; Aigueperse, J.; Souidi, M. Vitamin D metabolism impairment in the rat's offspring following maternal exposure to 137cesium. Arch. Toxicol. 2009, 83, 357-362.
Li, H.-B.; Xue, R.-Y.; Chen, X.-Q.; Lin, X.-Y.; Shi, X.-X.; Du, H.-Y.; Yin, N.-Y.; Cui, Y.-S.; Li, L.-N.; Scheckel, K.G.; et al. Ca Minerals and Oral Bioavailability of Pb, Cd, and As from Indoor Dust in Mice: Mechanisms and Health Implications. Environ. Health Perspect. 2022,130,127004.
Liu, D.Y.; Li, R.Y.; Fu, L.J.; Adu-Gyamfi, E.A.; Yang, Y.; Xu, Y.; Zhao, L.T.; Zhang, T.F.; Bao, H.Q.; Xu, X.O.; et al. SNP rs12794714 of CYP2R1 is associated with serum vitamin D levels and recurrent spontaneous abortion (RSA): A case-control study. Arch. Gynecol. Obstet. 2021, 304,179-190.
Wang, Y.; Wang, O.; Li, W.; Ma, L.; Ping, F.; Chen, L.; Nie, M. Variants in Vitamin D Binding Protein Gene Are Associated With Gestational Diabetes Mellitus. Medicine 2015, 94, e1693.
Strushkevich, N.; Usanov, S.A.; Plotnikov, A.N.; Jones, G.; Park, H.W. Structural analysis of CYP2R1 in complex with vitamin D3. J. Mol. Biol. 2008, 380, 95-106.
El-Boshy, M.; Refaat, B.; Almaimani, R.A.; Abdelghany, A.H.; Ahmad, J.; Idris, S.; Almasmoum, H.; Mahbub, A.A.; Ghaith, M.M.; BaSalamah, M.A. Vitamin D3 and calcium cosupplementation alleviates cadmium hepatotoxicity in the rat: Enhanced antioxidative and anti-inflammatory actions by remodeling cellular calcium pathways. J. Biochem. Mol. Toxicol. 2020, 34, e22440.
Permenter, M.G.; Dennis, W.E.; Sutto, T.E.; Jackson, D.A.; Lewis, J.A.; Stallings, J.D. Exposure to cobalt causes transcriptomic and proteomic changes in two rat liver derived cell lines. PLoS ONE 2013, 8, e83751.
Moore, L.E.; Karami, S.; Steinmaus, C.; Cantor, K.P. Use of OMIC technologies to study arsenic exposure in human populations. Environ. Mol. Mutagen. 2013, 54, 589-595.
Raza, A.; Tabassum, J.; Zahid, Z.; Charagh, S.; Bashir, S.; Barmukh, R.; Khan, R.S.A.; Barbosa, F., Jr.; Zhang, C.; Chen, H.; et al. Advances in "Omics" Approaches for Improving Toxic Metals/Metalloids Tolerance in Plants. Front. Plant Sci. 2022,12, 794373.
1. Vitamin D and Toxic Metals in Pregnancy - a Biological Perspective - June 2024Current Epidemiology Reports Volume 11, pages 153–163, (2024)
Mandy Fisher, Hope A. Weiler, Jordan R. Kuiper, Michael Borghese, Jessie P. Buckley, Robin Shutt, Jillian Ashley-Martin, Anita Subramanian, Tye E. Arbuckle, Beth K. Potter, Julian Little, Anne-Sophie Morisset & Anne Marie Jukic
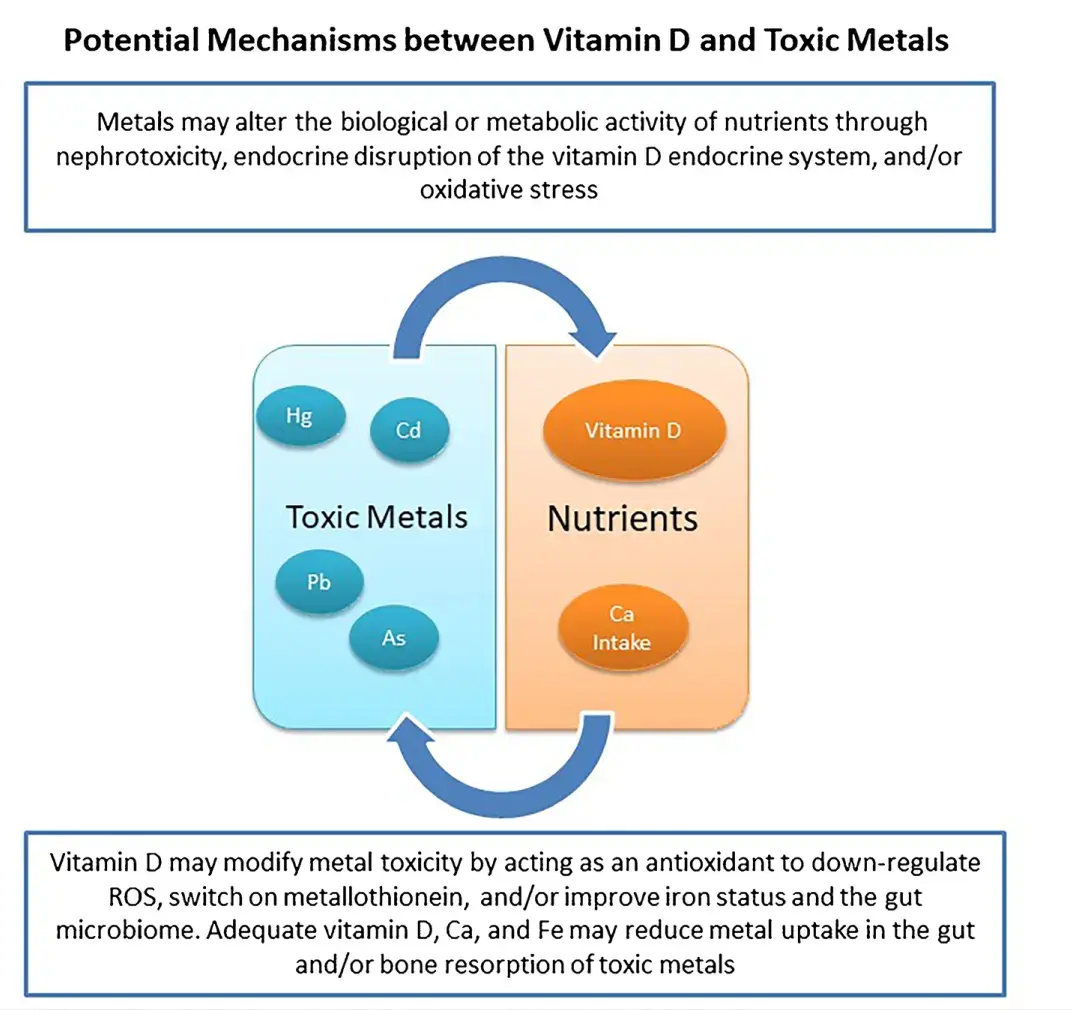
Purpose of Review
To discuss the potential biological mechanisms between vitamin D and toxic metals and summarize epidemiological studies examining this association in pregnant women.
Recent Findings
We identified four plausible mechanisms whereby vitamin D and toxic metals may interact: nephrotoxicity, intestinal absorption of metals, endocrine disruption, and oxidative stress. Few studies have examined the association between vitamin D and toxic metals in pregnant women. North American studies suggest that higher vitamin D status early in pregnancy are associated with lower blood metals later in pregnancy. However, a trial of vitamin D supplementation in a pregnant population, with higher metal exposures and lower overall nutritional status, does not corroborate these findings.
Summary
Given ubiquitous exposure to many toxic metals, nutritional intervention could be a means for prevention of adverse outcomes. Future prospective studies are needed to establish a causal relationship and clarify the directionality of vitamin D and metals.
1. 4 related items in Vitamin D Life