www.wikidoc.org/index.php/Vitamin_D July 2010
Vitamin D
You don't need to be Editor-In-Chief to add or edit content to WikiDoc. You can begin to add to or edit text on this WikiDoc page by clicking on the edit button at the top of this page. Next enter or edit the information that you would like to appear here. Once you are done editing, scroll down and click the Save page button at the bottom of the page.
| WikiDoc Resources for Vitamin D | |
| Articles | |
|---|---|
| Most recent articles on Vitamin D | |
| Media | |
| Evidence Based Medicine | |
| Clinical Trials | |
| Ongoing Trials on Vitamin D at Clinical Trials.gov Clinical Trials on Vitamin D at Google
| |
| Guidelines / Policies / Govt | |
| US National Guidelines Clearinghouse on Vitamin D
| |
| Books | |
| News | |
| Commentary | |
| Definitions | |
| Patient Resources / Community | |
| Patient resources on Vitamin D Discussion groups on Vitamin D Directions to Hospitals Treating Vitamin D Risk calculators and risk factors for Vitamin D
| |
| Healthcare Provider Resources | |
| Causes & Risk Factors for Vitamin D | |
| Continuing Medical Education (CME) | |
| International | |
|
| |
| Business | |
| Experimental / Informatics | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. 1 Phone:617-632-7753
Assistant Editor in Chief: Hector Tamez 2
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us 3 to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Vitamin D is a group of fat-soluble prohormones, the two major forms of which are vitamin D2 (or ergocalciferol) and vitamin D3 (or cholecalciferol).1 The term vitamin D also refers to metabolites and other analogues of these substances. Vitamin D3 is produced in skin exposed to sunlight, specifically ultraviolet B radiation.
Vitamin D plays an important role in the maintenance of organ systems.2
- Vitamin D regulates the calcium and phosphorus levels in the blood by promoting their absorption from food in the intestines, and by promoting re-absorption of calcium in the kidneys.
- It promotes bone formation and mineralization and is essential in the development of an intact and strong skeleton.
- It inhibits parathyroid hormone secretion from the parathyroid gland.
- Vitamin D affects the immune system by promoting immunosuppression, phagocytosis, and anti-tumor activity.
Vitamin D deficiency can result from inadequate intake coupled with inadequate sunlight exposure, disorders that limit its absorption, conditions that impair conversion of vitamin D into active metabolites, such as liver or kidney disorders, or, rarely, by a number of hereditary disorders.2 Deficiency results in impaired bone mineralization, and leads to bone softening diseases, rickets in children and osteomalacia in adults, and possibly contributes to osteoporosis. Vitamin D deficiency may also be linked to many forms of cancer.
Forms
Several forms of vitamin D have been described. The two major forms are vitamin D2 or ergocalciferol, and vitamin D3 or cholecalciferol.
- Vitamin D1: molecular compound of ergocalciferol with lumisterol, 1:1
- Vitamin D2: ergocalciferol or calciferol (made from ergosterol)
- Vitamin D3: cholecalciferol (made from 7-dehydrocholesterol in the skin).
- Vitamin D4: 22-dihydroergocalciferol
- Vitamin D5: sitocalciferol (made from 7-dehydrositosterol)
Chemically, the various forms of vitamin D are secosteroids; i.e. broken-open steroids.3 The structural difference between vitamin D2 and vitamin D3 is in their side chains. The side chain of D2 contains a double bond between carbons 22 and 23, and a methyl group on carbon 24.
Vitamin D2 is derived from fungal and plant sources, and is not produced by the human body. Vitamin D3 is derived from animal sources and is made in the skin when 7-dehydrocholesterol reacts with UVB ultraviolet light at wavelengths between 270–290 nm.4 These wavelengths are present in sunlight at sea level when the sun is more than 45° above the horizon, or when the UV index is greater than 3.5 At this solar elevation, which occurs daily within the tropics, daily during the spring and summer seasons in temperate regions, and almost never within the arctic circles, adequate amounts of vitamin D3 can be made in the skin only after ten to fifteen minutes of sun exposure at least two times per week to the face, arms, hands, or back without sunscreen. With longer exposure to UVB rays, an equilibrium is achieved in the skin, and the vitamin simply degrades as fast as it is generated.1
In most mammals, including humans, D3 is more effective than D2 at increasing the levels of vitamin D hormone in circulation; D3 is at least 3-fold, and likely closer to 10-fold, more potent than D2.6 However, in some species, such as rats, vitamin D2 is more effective than D3.7 Both vitamin D2 and D3 are used for human nutritional supplementation, and pharmaceutical forms include calcitriol (1alpha, 25-dihydroxycholecalciferol), doxercalciferol and calcipotriene.8
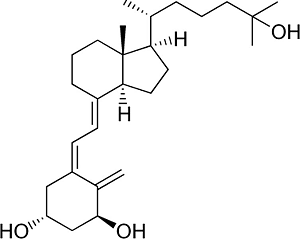
Some forms of activated vitamin D like calcitriol, that are commonly used in the chronic kidney disease population, can cause increase absorption of calcium and phosphorus in the gut leading to high serum levels. Some companies modified the structures of the side-chain decreasing the gut effect and maintaining the effect in the parathyroid gland.9 These group of compounds includes paricalcitol and the one named after Hector DeLuca, hectorol.
Biochemistry
Vitamin D is a prohormone, that is, it has no hormone activity itself, but is converted to a hormone 1,25-D which does, through a tightly regulated synthesis mechanism.
Production in the skin
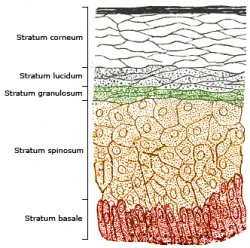
The skin consists of two primary layers: the inner layer called the dermis, composed largely of connective tissue, and the outer thinner epidermis. The epidermis consists of five strata; from outer to inner they are: the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale.
Vitamin D3 is produced photochemically in the skin from 7-dehydrocholesterol. The highest concentrations of 7-dehydrocholesterol are found in the epidermal layer of skin, specifically in the stratum basale and stratum spinosum.4 The production of pre-vitamin D3 is therefore greatest in these two layers, whereas production in the other layers is reduced.
Synthesis in the skin involves UVB radiation which effectively penetrates only the epidermal layers of skin. 7-Dehydrocholesterol absorbs UV light most effectively at wavelengths between 270–290 nm and thus the production of vitamin D3 will only occur at those wavelengths. The two most important factors that govern the generation of pre-vitamin D3 are the quantity (intensity) and quality (appropriate wavelength) of the UVB irradiation reaching the 7-dehydrocholesterol deep in the stratum basale and stratum spinosum.4
A critical determinant of vitamin D3 production in the skin is the presence and concentration of melanin. Melanin functions as a light filter in the skin, and therefore the concentration of melanin in the skin is related to the ability of UVB light to penetrate the epidermal strata and reach the 7-dehydrocholesterol-containing stratum basale and stratum spinosum. Under normal circumstances, ample quantities of 7-dehydrocholesterol (about 25-50 mg/cm² of skin) are available in the stratum spinosum and stratum basale of human skin to meet the body's vitamin D requirements,4 and melanin content does not alter the amount of vitamin D that can be produced.10 Thus, individuals with higher skin melanin content will simply require more time in sunlight to produce the same amount of vitamin D as individuals with lower melanin content.
Synthesis mechanism (form 3)
| 1. Vitamin D3 is synthesized from 7-dehydrocholesterol, a derivative of cholesterol, which is then photolyzed by ultraviolet light in 6-electron conrotatory electrocyclic reaction. The product is pre-vitamin D3. | 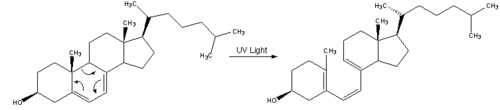 |
| 2. Pre-vitamin D3 then spontaneously isomerizes to Vitamin D3 in a antarafacial hydride 1,7Sigmatropic shift. |  |
| 3. Whether it is made in the skin or ingested, vitamin D3 (cholecalciferol) is then hydroxylated in the liver to 25-hydroxycholecalciferol (25(OH)D3 or calcidiol) by the enzyme 25-hydroxylase produced by hepatocytes, and stored until it is needed. 25-hydroxycholecalciferol is further hydroxylated in the kidneys by the enzyme 1?-hydroxylase, into two dihydroxylated metabolites, the main biologically active hormone 1,25-dihydroxycholecalciferol (1,25(OH)2D3 or calcitriol) and 24R,25(OH)2D3. This conversion occurs in a tightly regulated fashion. Calcitriol is represented below right (hydroxylated Carbon 1 is on the lower ring at right, hydroxylated Carbon 25 is at the upper right end). | 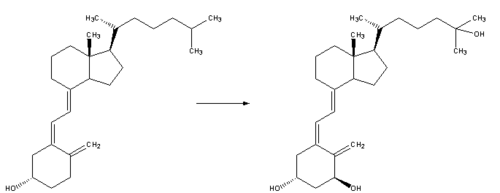 |
Mechanism of action
Once vitamin D is produced in the skin or consumed in food, it is converted in the liver and kidney to form 1,25 dihydroxyvitamin D, (1,25(OH)2D) the physiologically active form of vitamin D (when "D" is used without a subscript it refers to either D2 or D3). Following this conversion, the hormonally active form of vitamin D is released into the circulation, and by binding to a carrier protein in the plasma, vitamin D binding protein (VDBP), it is transported to various target organs.3
The hormonally active form of vitamin D mediates its biological effects by binding to the vitamin D receptor (VDR), which is principally located in the nuclei of target cells.3 The binding of calcitriol to the VDR allows the VDR to act as a transcription factor that modulates the gene expression of transport proteins (such as TRPV6 and calbindin), which are involved in calcium absorption in the intestine.
The Vitamin D receptor belongs to the nuclear receptor superfamily of steroid/thyroid hormone receptors, and VDR are expressed by cells in most organs, including the brain, heart, skin, gonads, prostate, and breast. VDR activation in the intestine, bone, kidney, and parathyroid gland cells leads to the maintenance of calcium and phosphorus levels in the blood (with the assistance of parathyroid hormone and calcitonin) and to the maintenance of bone content.11
The VDR is known to be involved in cell proliferation, differentiation. Vitamin D also affects the immune system, and VDR are expressed in several white blood cells including monocytes and activated T and B cells.8
Nutrition
Very few foods are naturally rich in vitamin D, and most vitamin D intake is in the form of fortified products including milk, soy milk and cereal grains.1
A blood calcidiol (25-hydroxy-vitamin D) level is the accepted way to determine vitamin D nutritional status. The optimal level of serum 25-hydroxyvitamin D remains a point for debate among medical scientists.
The U.S. Dietary Reference Intake for Adequate Intake (AI) of vitamin D for infants, children and men and women aged 19–50 is 5 micrograms/day (200 IU/day).12 Adequate intake increases to 10 micrograms/day (400 IU/day) for men and women aged 51–70 and to 15 micrograms/day (600 IU/day) past the age of 70.1
In food
Season, geographic latitude, time of day, cloud cover, smog, and sunscreen affect UV ray exposure and vitamin D synthesis in the skin, and it is important for individuals with limited sun exposure to include good sources of vitamin D in their diet.
In some countries, foods such as milk, yogurt, margarine, oil spreads, breakfast cereal, pastries, and bread are fortified with vitamin D2 and/or vitamin D3, to minimize the risk of vitamin D deficiency.13 In the United States and Canada, for example, fortified milk typically provides 100 IU per glass, or one quarter of the estimated adequate intake for adults over the age of 50.1
Fortified foods represent the major dietary sources of vitamin D, as very few foods naturally contain significant amounts of vitamin D.
Natural sources of vitamin D include:1
- Fish liver oils, such as cod liver oil, 1 Tbs. (15 mL) provides 1,360 IU
- Fatty fish species, such as:
- Mushrooms provide over 2700 IU per serving (approx. 3 oz or 1/2 cup) of vitamin D2, if exposed to just 5 minutes of UV light after being harvested;14 this is one of a few natural sources of vitamin D for vegans.
- One whole egg, 20 IU
- Yeast
Deficiency
There is no consensus on the serum 25-hydroxyvitamin D levels considered "normal". However, most experts would agree that a value equal or less than 20 ng per milliliter (50 nmol per liter) is in the deficient range.15,16,17,18 Other people consider the point at which the parathyroid hormone (PTH) levels off as the cutoff point, which is close to 30 ng per milliliter (75 nmol per liter). 19 Vitamin D deficiency can result from: inadequate intake coupled with inadequate sunlight exposure, disorders that limit its absorption, conditions that impair conversion of vitamin D into active metabolites, such as liver or kidney disorders, or, rarely, by a number of hereditary disorders.2 Deficiency results in impaired bone mineralization, and leads to bone softening diseases, rickets in children and osteomalacia in adults, and possibly contributes to osteoporosis.2
Diseases caused by deficiency
The role of diet in the development of rickets was determined by Edward Mellanby between 1918–1920.20 In 1921 Elmer McCollum identified an anti-rachitic substance found in certain fats could prevent rickets. Because the newly discovered substance was the fourth vitamin identified, it was called vitamin D.20 The 1928 Nobel Prize in Chemistry was awarded to Adolf Windaus, who discovered the steroid, 7-dehydrocholesterol, the precursor of vitamin D.
Vitamin D deficiency is known to cause several bone diseases21 including:
- Rickets, a childhood disease characterized by impeded growth, and deformity, of the long bones.
- Osteomalacia, a bone-thinning disorder that occurs exclusively in adults and is characterised by proximal muscle weakness and bone fragility.
- Osteoporosis, a condition characterized by reduced bone mineral density and increased bone fragility.
Prior to the fortification of milk products with vitamin D, rickets was a major public health problem. In the United States, milk has been fortified with 10 micrograms (400 IU) of vitamin D per quart since the 1930s, leading to a dramatic decline in the number of rickets cases.11
Vitamin D malnutrition may also be linked to an increased susceptibility to several chronic diseases such as high blood pressure, tuberculosis, cancer, periodontal disease, multiple sclerosis, chronic pain, depression, schizophrenia, seasonal affective disorder and several autoimmune diseases (see role in immunomodulation).11
Groups at greater risk of deficiency
Vitamin D requirements increase with age, while the ability of skin to convert 7-dehydrocholesterol to pre-vitamin D3 decreases.22 In addition the ability of the kidneys to convert calcidiol to its active form also decreases with age, prompting the need for increased vitamin D supplementation in elderly individuals. One consensus concluded that for optimal prevention of osteoporotic fracture the blood calcidiol concentration should be higher than 30 ng/mL, which is equal to 75 nmol/L.23
The American Pediatric Associations advises vitamin D supplementation of 200 IU/day (5?g/d) from birth onwards.1 Health Canada recommends 400IU/day (10?g/d).24 While infant formula is generally fortified with vitamin D, breast milk does not contain significant levels of vitamin D, and parents are usually advised to avoid exposing babies to prolonged sunlight. Therefore, infants who are exclusively breastfed are likely to require vitamin D supplementation beyond early infancy, especially at northern latitudes.24 Liquid "drops" of vitamin D, as a single nutrient or combined with other vitamins, are available in water based or oil-based preparations ("Baby Drops" in North America, or "Vigantol oil" in Europe). However, babies may be safely exposed to sunlight for short periods; as little as 10 minutes a day without a hat can suffice, depending on location and season. The vitamin D found in supplements and infant formula is less easily absorbed than that produced by the body naturally and carries a risk of overdose that is not present with natural exposure to sunlight.
Obese individuals may have lower levels of the circulating form of vitamin D, probably because of reduced bioavailability, and are at higher risk of deficiency. To maintain blood levels of calcium, therapeutic vitamin D doses are sometimes administered (up to 100,000 IU or 2.5 mg daily) to patients who have had their parathyroid glands removed (most commonly renal dialysis patients who have had tertiary hyperparathyroidism, but also to patients with primary hyperparathyroidism) or with hypoparathyroidism.25 Patients with chronic liver disease or intestinal malabsorption disorders may also require larger doses of vitamin D (up to 40,000 IU or 1 mg (1000 micrograms) daily).
The use of sunscreen with a sun protection factor (SPF) of 8 inhibits more than 95% of vitamin D production in the skin.1126 Recent studies showed that, following the successful "Slip-Slop-Slap" health campaign encouraging Australians to cover up when exposed to sunlight to prevent skin cancer, an increased number of Australians and New Zealanders became vitamin D deficient.13 Ironically, there are indications that vitamin D deficiency may lead to skin cancer.27 To avoid vitamin D deficiency dermatologists recommend supplementation along with sunscreen use.
The reduced pigmentation of light-skinned individuals tends to allow more sunlight to be absorbed even at higher latitudes, thereby reducing the risk of vitamin D deficiency.23 However, at higher latitudes (above 30°) during the winter months, the decreased angle of the sun's rays, reduced daylight hours, protective clothing during cold weather, and fewer hours of outside activity, diminish absorption of sunlight and the production of vitamin D. Because melanin acts like a sun-block, prolonging the time required to generate vitamin D, dark-skinned individuals, in particular, may require extra vitamin D to avoid deficiency at higher latitudes. At latitudes below 30° where sunlight and day-length are more consistent, vitamin D supplementation may not be required.5 Individuals clad in full body coverings during all their outdoor activity, most notably women wearing burquas in daylight, are at risk of vitamin D deficiency. This poses a lifestyle-related health risk mostly for female residents of conservative Muslim nations in the Middle East, but also for strict adherents in other parts of the world.28
Overdose
- For more details on this topic, see hypervitaminosis D.
Vitamin D stored in the human body as calcidiol (25-hydroxy-vitamin D) has a large volume of distribution and a long half-life (about 20 to 29 days).8 However, the synthesis of bioactive vitamin D hormone is tightly regulated and vitamin D toxicity usually occurs only if excessive doses (prescription or megavitamin) are taken.29 Although normal food and pill vitamin D concentration levels are too low to be toxic in adults, because of the high vitamin A content in codliver oil it is possible to reach poisonous levels of vitamin A,30 if taken in multiples of the normal dose in an attempt to increase the intake of vitamin D. Most cases of vitamin D overdose have occurred due to manufacturing and industrial accidents.31
Exposure to sunlight for extended periods of time does not cause Vitamin D toxicity.31 This is because within about 20 minutes of ultraviolet exposure in light skinned individuals (3–6 times longer for pigmented skin) the concentration of vitamin D precursors produced in the skin reach an equilibrium, and any further vitamin D that is produced is degraded.32 Maximum endogenous production with full body exposure to sunlight is 250 µg (10,000 IU) per day.31
The exact long-term safe dose of vitamin D is not entirely known, but dosages up to 60 micrograms (2,400 IU) /day in healthy adults are believed to be safe.8, and all known cases of vitamin D toxicity with hypercalcemia involve intake of or over 1,000 micrograms (40,000 IU)/day31. The U.S. Dietary Reference Intake Tolerable Upper Intake Level (UL) of vitamin D for children and adults is 50 micrograms/day (2,000 IU/day). In adults, sustained intake of 2500 ?g/day (100,000 IU) can produce toxicity within a few months.2 For infants (birth to 12 months) the tolerable UL is set at 25 micrograms/day (1000 IU/day), and vitamin D concentrations of 1000 micrograms/day (40,000 IU) in infants has been shown to produce toxicity within 1 to 4 months. In the United States, overdose exposure of vitamin D was reported by 284 individuals in 2004, leading to 1 death.33
Serum levels of calcidiol (25-hydroxy-vitamin D) are typically used to diagnose vitamin D overdose. In healthy individuals, calcidiol levels are normally between 25 to 40 ng/mL (60 to 100 nmol/L), but these levels may be as much as 15-fold greater in cases of vitamin D toxicity. Serum levels of bioactive vitamin D hormone (1,25(OH2)D) are usually normal in cases of vitamin D overdose.2
The symptoms of vitamin D toxicity are a result of hypercalcemia (an elevated level of calcium in the blood) caused by increased intestinal calcium absorption. Gastrointestinal symptoms of vitamin D toxicity can develop including anorexia, nausea, and vomiting. These symptoms are often followed by polyuria (excessive production of urine), polydipsia (increased thirst), weakness, nervousness, pruritus (itch), and eventually renal failure. Other signals of kidney disease including elevated protein levels in the urine, urinary casts, and a build up of wastes in the blood stream can also develop.2 In one study, hypercalciuria and bone loss occurred in four patients with documented vitamin D toxicity.34 Another study showed elevated risk of ischaemic heart disease when 25D was above 89 ng/mL.35
Vitamin D toxicity is treated by discontinuing vitamin D supplementation, and restricting calcium intake. If the toxicity is severe blood calcium levels can be further reduced with corticosteroids or bisphosphonates. In some cases kidney damage may be irreversible.2
Role in immunomodulation
The hormonally active form of vitamin D mediates immunological effects by binding to nuclear vitamin D receptors (VDR) which are present in most immune cell types including both innate and adaptive immune cells. The VDR is expressed constitutively in monocytes and in activated macrophages, dendritic cells, NK cells, T and B cells. In line with this observation, activation of the VDR has potent anti-proliferative, pro-differentiative, and immunomodulatory functions including both immune-enhancing and immunosuppressive effects.36
Effects of VDR-ligands, such as vitamin D hormone, on T-cells include suppression of T cell activation and induction of regulatory T cells, as well as effects on cytokine secretion patterns.37 VDR-ligands have also been shown to affect maturation, differentiation, and migration of dendritic cells, and inhibits DC-dependent T cell activation, resulting in an overall state of immunosuppression.38
VDR ligands have also been shown to increase the activity of natural killer cells, and enhance the phagocytic activity of macrophages.8 Active vitamin D hormone also increases the production of cathelicidin, an antimicrobial peptide that is produced in macrophages triggered by bacteria, viruses, and fungi.39 Vitamin D deficiency tends to increase the risk of infections, such as influenza and tuberculosis. In a 1997 study, Ethiopian children with rickets were 13 times more likely to get pneumonia than children without rickets.40
These immunoregulatory properties indicate that ligands with the potential to activate the VDR, including supplementation with calcitriol (as well as a number of synthetic modulators), may have therapeutic clinical applications in the treatment of; inflammatory diseases (rheumatoid arthritis, psoriatic arthritis), dermatological conditions (psoriasis, actinic keratosis), osteoporosis, cancers (prostate, colon, breast, myelodysplasia, leukemia, head and neck squamous cell carcinoma, and basal cell carcinoma), and autoimmune diseases (systemic lupus erythematosus, type I diabetes, multiple sclerosis) and in preventing organ transplant rejection.36 However the effects of supplementation with vitamin D, as yet, remain unclear, and supplementation may be inadvisable for individuals with sarcoidosis and other diseases involving vitamin D hypersensitivity.413142
A 2006 study published in the Journal of the American Medical Association, reported evidence of a link between Vitamin D deficiency and the onset of Multiple Sclerosis; the authors posit that this is due to the immune-response suppression properties of Vitamin D.43
Role in cancer prevention and recovery
The vitamin D hormone, calcitriol, has been found to induce death of cancer cells in vitro and in vivo. Although the anti-cancer activity of vitamin D is not fully understood, it is thought that these effects are mediated through vitamin D receptors expressed in cancer cells, and may be related to its immunomodulatory abilities. The anti-cancer activity of vitamin D observed in the laboratory has prompted some to propose that vitamin D supplementation might be beneficial in the treatment or prevention of some types of cancer.8
In 2005, scientists released a metastudy which demonstrated a beneficial correlation between vitamin D intake and prevention of cancer. Drawing from a meta-analysis of 63 published reports, the authors showed that intake of an additional 1,000 international units (IU) (or 25 micrograms) of vitamin D daily reduced an individual's colon cancer risk by 50%, and breast and ovarian cancer risks by 30%.44 Research has also shown a beneficial effect of high levels of calcitriol on patients with advanced prostate cancer.45 A randomised intervention study involving 1,200 women, published in June 2007, reports that vitamin D supplementation (1,100 international units (IU) / day) resulted in a 60% reduction in cancer incidence, during a four-year clinical trial, rising to a 77% reduction if cancers diagnosed in the first year (and therefore more likely to have originated prior to the intervention) were excluded.4647 A recent study using data on over 4 million cancer patients from 13 different countries showed a marked difference in cancer risk between countries classified as sunny and countries classified as less–sunny for a number of different cancers.48
In June 2007, The Canadian Cancer Society began recommending that all adult Canadians consider taking 1000 IU of vitamin D during the fall and winter months (when typically the country's northern latitude prevents sufficient sun-stimulated production of vitamin D). This kind of recommendation is a first for cancer agencies.49
Research has also suggested that cancer patients who have surgery or treatment in the summer — and therefore make more endogenous vitamin D — have a better chance of surviving their cancer than those who undergo treatment in the winter when they are exposed to less sunlight.50
Role in cardiovascular diseases
History
One of the very first indications of the association between cardiovascular diseases and vitamin D was in the most severe form of the vitamin deficiency, Rickets, in which patients had cardiomyopathy.51 Evidence of an intracellular vitamin D receptor in rat cardiomyocytes was described in the early 1980's52 and in human heart tissue in the mid 1990s.53 Xiang W. et al. provided further evidence of the relationship between vitamin D and cardiovascular diseases in his VDR knockout mouse model, in which the mice had severe cardiac hypertrophy.54 VDR knockout mice also under-express tissue inhibitors of metalloproteinases (TIMP-1 & TIMP-3) in comparison to the wild-type, which may contribute to increased oxidation of the extracellular matrix proteins and perivascular tissues.55 Interestingly, spontaneous hypertensive rats (SHR) were found to be more vitamin D deficient as measures of blood pressure increased.56 Contrary to data in the vitamin D receptor knockout mice model, the wild-type had higher blood pressures compared to the knockout.57
In vitro and animal studies
In vitro data showed that 1,25-hydroxyvitamin D has a regulatory effect on myoblast proliferation as well as some rapid genomic effect, suggesting some action independent of transcription.58 To further explore possible non-genomic effects, Tishkoff et al. explored the location of the VDR intracellularly and found signaling in the T-tubules, close to the sarcoendoplasmic reticulum Ca++- ATPase (SERCA).59 They also reported accelerated rates of myocyte contraction and relaxation in VDR knockout mice cells as compared to the wild-type.
Dahl salt-sensitive rats (DSS) are an interesting animal model to study direct cardiac vitamin D effects on the heart since the DSS rats become vitamin D deficient, hypertensive and develop increased heart mass if fed with high-salt diet. Bodyak et al. showed decreased heart size and improved contraction by m-mode echocardiography in rats treated with paricalcitol versus rats treated with placebo.60 Atrial natriuretic factor (ANP) was lower in the treated group, findings which are similar to those previously described in cardiomyocyte cell cultures.61 There were differences shown in the gene expression profiles between DSS treated with paricalcitol versus vehicle.
Observational studies
Multiple epidemiologic studies have shown improved in cardiovascular morbidity and mortality in several populations. One of the earliest associations was shown by Teng et al. in the New England Journal of Medicine in 2003, where they showed a benefit in survival independent of calcium, phosphorus and PTH for patients treated with activated vitamin D.62 There was controversy regarding the activated vitamin D effects in mortality, because of higher risk of hypercalcemia, hyperposphatemia and their effect in the cardiovascular system. Patients treated with Paricalcitol, an activated vitamin D analogue with less effect on calcium and phosphorus, had improved survival compared to those treated with calcitriol. In further studies in the chronic kidney disease population, the vitamin D level was shown to be predictive of 90-day all-cause and cardiovascular mortality63 and the administration of activated form of vitamin D was shown to improve survival.64 Recently, in the New England Journal of Medicine Dr. Lee et al. described how the sicker patients in multiple intesive care units have lower levels of vitamin D.65
There is discrepancy in the prevalence and in the response to treatment of cardiovascular diseases among different races and ethnicities. In the third National Health and Nutrition Examination Survey (NHANES) black people, who have higher cardiovascular risk compared to whites, had lower levels of 25-hydroxyvitamin D.66 Moreover, multiple risk factors such as obesity, hypertension and diabetes mellitus were associated with lower 25-hydroxyvitamin D levels. In other studies 25-hydroxyvitamin D levels were inversely correlated with the prevalence of metabolic syndrome,67 type 2 diabetes mellitus68 and hypertension.69 In a cohort study with more than five thousand participants that were followed for twenty years the cumulative incidence of heart failure in black woman was 1.1% and in black men 0.9% compared to 0.8% and 0% in white women and men respectively.70 Interestingly, paradoxically to other scenarios, the african-american population has a survival advantage compared to whites in hemodialysis. Wolf et al. showed that they are more likely to be treated with activated vitamin D.71 Therefore, if you compare african-american versus whites in the non-treated population their survival advantage is lost.
Zittermann et al. demonstrated that geographic factors such as latitude, altitude, season and place of residency were associated with different cardiovascular risk, mostly for ischemic heart disease.72 Interestingly, those factors were also associated with 25-hydroxyvitamin D levels. Cardiovascular disease rates were at their zenith in the winter, when the vitamin D levels were at their nadir. Based on studies done by Holick, PhD, MD, we know that the prevalence of vitamin D deficiency is close to somewhere between 40-100% in healthy individuals at the end of the winter in the New England area.73 Similar seasonal variations have also been shown for heart failure hospitalization rates74 and in cerebrovascular disease.75
The Ludwigshafen Risk and Cardiovascular Health Study (LURIC), a prospective cohort design to genetic polymorphisms and biomarkers associated with cardiovascular risks, showed a correlation between 25-hydroxyvitamin D and C-reactive protein (CRP) and interleukin 6 levels (IL-6), serum phospholipid and gluthatione levels and vascular cell adhesion molecule 1 (VCAM1) and intracellular adhesion molecule 1 (ICAM1).76 Dobnig et al. showed that 25-hydroxyvitamin D levels had a significant hazard ratio of 2.08 for cardiovascular mortality comparing the lowest versus the highest quartiles with a follow up close to eight years, and 1.61 for 1,25-hydroxyvitamin D. Results suggest independent predictive value of 25-hydroxyvitamin D and 1,25-hydroxyvitamin D. The results were independent of coronary artery disease, physical activity level, Charlson Comorbidity Index, variables of mineral metabolism and New York Heart Association functional class. The hazard ratios increased progressively per lower vitamin D level, suggesting a dose-dependent effect and strengthening the association. However, the study assumed that the patients with non-cardiac death had the same probability of dying of cardiac etiologies than the rest of the population, which may not necessarily be true and may bias the estimates.
Research indicates that vitamin D plays a role in preventing or reversing coronary disease. As with cancer incidence, the same qualitative inverse correlations exist between coronary disease incidence and serum vitamin D levels,77 seasonal solar exposure,78 in temperate latitudes79 but not tropical latitudes.80
A nested case-control study with 18,225 men in the Health Professionals Follow-up Study showed that vitamin D deficient people had a relative risk of 2.09 of developing myocardial infarction.81 Similar findings were also shown in the researchers from the Framingham Offspring study prospectively collected 25-OH D levels in 1739 white individuals without cardiovascular disease or renal disease. For the primary analysis, vitamin D levels were a categorical variable, with cut-offs chosen a priori based on previously published studies. Participants were classified as deficient (<15 ng/ml) or sufficient (>15 ng/ml). Mean level was 19.7 ng/ml among all participants. Low 25-OH D levels were associated with obesity, cigarette smoking, higher systolic blood pressure, diabetes, higher ratio of total to HDL cholesterol, and lower intake of vitamin D or vitamins. Interesting, there was no correlation with physical activity.
Over a mean of 7.6 years follow up there were 120 cardiovascular events. A multivariate model was used to correlate 25-OH D status with cardiovascular risk, adjusting for age, sex, blood pressure, medications, diabetes, cigarette smoking, cholesterol, body mass index, and renal function. After adjustment, a 25-OH D level less than 15 ng/ml was independently associated with risk of a cardiovascular event (HR 1.62, CI 1.11 to 2.35, P=0.01). In a three-category model, where participants were classified as sufficient, deficient (10 to 15 ng/ml) or severely deficient (<10 ng/ml), severe deficiency was associated with even greater risk (HR 1.80, CI 1.05 to 3.08, P=0.01).
The mechanism by which vitamin D deficiency might increase risk of vascular disease is not entirely known. The authors cite data demonstrating that 1,25-OH D is important for suppression of the renin-angiotensin axis, and possible effects of hyperparathyroidism on myocyte function and vascular inflammation. The possibility always exists that an unmeasured confounder might explain the correlation seen in this study. Nonetheless, it is telling that the majority of participants in this study would qualify as vitamin-D deficient if a cut-off of 20 ng/ml were used, and the body of evidence linking vitamin-D deficiency with chronic illness is impressive.
Observational data has shown similar associations of vitamin D not only with coronary artery disease, but with equivalents such as peripheral vascular disease.82 Differences by race in PVD have also been reported.83
A retrospective pilot study of hemodialysis patients demonstrated improved diastolic function evaluated with lower E/A ratios on echocardiogram in patients treated with paricalcitol as compared to untreated patients.84 A cohort of 51 patients was enrolled and followed for 12 months. Treatment was left at discretion of nephrologists. Baseline and 12-month echocardiograms were compared.
Randomized clinical trials
In a very small and limited clinical trial in Korea, 25 patients with end-stage renal disease on hemodialysis and secondary hyperparathyroidism received either received calcitriol or nothing.85 The treatment group M-mode echocardiography showed pronounced reductions in inverventricular wall thickness, left ventricular posterior wall thickness and left ventricle mass index. There were no differences in blood pressure control, and blood pressure medications were not changed. Data regarding distribution of angiotensin converting enzyme inhibitors and angiotensin receptor blockers between groups is not shown. Plasma renin, angiotensin II and atrial natriuretic peptide decreased over time in the calcitriol group. The study is limited due to its sample size, because it does not specify why some patients received treatment and why some did not, and it does not show the hormonal change in the control group.
There are two large randomized double-blind placebo-controlled trials in chronic kidney disease assessing the effect of paricalcitol treatment on diastolic dysfunction, left ventricular mass as well as biomarkers and genetic expression (http://clinicaltrials.gov/ct2/show/NCT00497146 and http://clinicaltrials.gov/ct2/show/NCT00616902 ). These trials promise rigorous data to further characterize the effect, and possible mechanisms of the effect, of activated vitamin D in the heart.
Notes and references
- ? 1.01.11.21.31.41.51.6Dietary Supplement Fact Sheet: Vitamin D. National Institutes of Health. Archived from the original on 2007-09-10. Retrieved on 2007-09-10.
- ? 2.02.12.22.32.42.52.62.7Vitamin D The Merck Manual of Diagnosis and Therapy. Last modified November 2005
- ? 3.03.13.2About Vitamin D Including Sections: History, Nutrition, Chemistry, Biochemistry, and Diseases. University of California Riverside
- ? 4.04.14.24.3 Norman, Anthony W. (1998) Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D:integral components of the vitamin D endocrine system. Am J Clin Nutr;67:1108–10.
- ? 5.05.1Fun with UVB Includes calculations and measurements of UVB levels at various angles of solar rays.
- ? Laura A. G. Armas, Bruce W. Hollis and Robert P. Heaney (2004). "Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans Full Text". The Journal of Clinical Endocrinology & Metabolism 89 (11): 5387–5391.
- ? Coates, M. E. (1968). "Requirements of different species for vitamins Full Text-pdf". Proceedings of the Nutrition Society 27 (2): 143–148. PMID 5755261.
- ? 8.08.18.28.38.48.5Vitamin D The Physicians Desk Reference. 2006 Thompson Healthcare.
- ? Slatopolsky E, Dusso A, Brown A (December 1999). "New analogs of vitamin D3". Kidney Int. Suppl. 73: S46–51. PMID 10633464.
- ? Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol 1991;127:536–8.
- ? 11.011.111.211.3Holick MF (2004). "Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease". American Journal of Clinical Nutrition Full Text 80 (6): 1678S-1688S.
- ? In scientific literature, vitamin D dosage in usually reported in micrograms, whereas food and supplement regulations typically require dosages on labels to be in International Units (IU). 1 microgram vitamin D equals 40 IU vitamin D.
- ? 13.013.1Nowson C, Margerison C (2002). "Vitamin D intake and vitamin D status of Australians". Med J Aust 177 (3): 149-52. PMID 12149085.
- ? "Bringing Mushrooms Out of the Dark", MSNBC, April 18 2006. Retrieved on 2007-08-06.
- ? Thomas MK, Lloyd-Jones DM, Thadhani RI, et al (March 1998). "Hypovitaminosis D in medical inpatients". N. Engl. J. Med. 338 (12): 777–83. PMID 9504937.
- ? Holick MF (March 2006). "High prevalence of vitamin D inadequacy and implications for health". Mayo Clin. Proc. 81 (3): 353–73. PMID 16529140.
- ? Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (July 2006). "Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes". Am. J. Clin. Nutr. 84 (1): 18–28. PMID 16825677.
- ? Malabanan A, Veronikis IE, Holick MF (March 1998). "Redefining vitamin D insufficiency". Lancet 351 (9105): 805–6. PMID 9519960.
- ? Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R (July 2005). "Estimates of optimal vitamin D status". Osteoporos Int 16 (7): 713–6. doi:10.1007/s00198-005-1867-7. PMID 15776217.
- ? 20.020.1Rajakumar K (2003). "Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective". Pediatrics 112 (2): e132-5. PMID 12897318.
- ? Grant WB, Holick MF (2005). "Benefits and requirements of vitamin D for optimal health: a review". Altern Med Rev 10 (2): 94-111. PMID 15989379.
- ? "Low vitamin D levels linked to poor physical performance in older adults", EurekAlert, April 23 2007. Retrieved on 2007-04-24.
- ? 23.023.1Heaney RP (2004). "Functional indices of vitamin D status and ramifications of vitamin D deficiency Full Text". Am J Clin Nutr 80 (6 Suppl): 1706S-9S.
- ? 24.024.1Vitamin D Supplementation for Breastfed Infants - 2004 Health Canada Recommendation
- ? Holick MF (2005). "The vitamin D epidemic and its health consequences Full Text". J Nutr 135 (11): 2739S-48S.
- ? Sayre, Robert M.; John C. Dowdy (2007). "Darkness at Noon: Sunscreens and Vitamin D3". Photochemistry and Photobiology 83 (2): 459. doi:10.1562/2006-06-29-RC-956.
- ? Grant WB (2002). "An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation". Cancer 94 (6): 1867-75. PMID 11920550.
- ? Saadi HF, Dawodu A, Afandi BO, Zayed R, Benedict S, Nagelkerke N (2007). "Efficacy of daily and monthly high-dose calciferol in vitamin D-deficient nulliparous and lactating women". Am. J. Clin. Nutr. 85 (6): 1565-71. PMID 17556694.
- ? RODENTICIDES, source: Journal of Veterinary Medicine, archives, vol. 27, May, 1998. IPM Of Alaska, Solving Pest Problems Sensibly. Retrieved on 2006-07-07.
- ? Bendich A, Langseth L (1989). "J Clin Nutr Safety of vitamin A" 49 (2): 358-71. PMID 2492745.
- ? 31.031.131.231.331.4Vieth R (1999). "Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety". Am J Clin Nutr 69 (5): 842-56. PMID 10232622.
- ? Holick M (1995). "Environmental factors that influence the cutaneous production of vitamin D". Am J Clin Nutr 61 (3 Suppl): 638S-645S. PMID 7879731.
- ? 2004 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System.
- ? Adams JS, Lee G (1997). "Gains in bone mineral density with resolution of vitamin D intoxication". Ann Intern Med 127 (3): 203-206. PMID 9245225.
- ? Rajasree S, Rajpal K, Kartha CC, Sarma PS, Kutty VR, Iyer CS, Girija G (2001). "Serum 25-hydroxyvitamin D3 levels are elevated in South Indian patients with ischemic heart disease Full Text". Eur J Epidemiol 17 (6): 567-71. PMID 11949730.
- ? 36.036.1 Nagpal, Sunil, Songqing Naand and Radhakrishnan Rathnachalam (2005) Noncalcemic Actions of Vitamin D Receptor Ligands Full Text Endocrine Reviews 26 (5): 662-687.
- ? Yee YK, Chintalacharuvu SR, Lu J, Nagpal S. (2005). "Vitamin D receptor modulators for inflammation and cancer.". Mini Rev Med Chem. 5 (8): 761–78. PMID 16101412.
- ? van Etten E, Mathieu C. (2005). "Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts.". J Steroid Biochem Mol Biol. 97 (1-2): 93–101. PMID 16046118.
- ? Janet Raloff, The Antibiotic Vitamin Science News, Vol 170, November 11, 2006, pages 312-317
- ? Muhe, L., et al., Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet (June 21, 1997) 349, 1801-1804. PMID 9269215
- ? United Kingdom Food Standards Agency; Expert Group on Vitamins and Minerals; Professor Michael Langdon, Chairman. (2003 May). Safe Upper Levels for Vitamins and Minerals. Retrieved Aug. 12, 2006 from http://www.food.gov.uk/multimedia/pdfs/vitmin2003.pdf
- ? Abreu MT, et al. (2004). "Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density". Gut 53 (8): 1129-1136. PMID 15247180.
- ? Munger KL. , Levin, LI,Hollis BW , Howard, NS , Ascherio A (2006). "Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis.". Journal of the American Medical Association 296 (23): 2832-2838. PMID 17179460.
- ? "Vitamin D 'can lower cancer risk'", BBC News, 28 December 2005. Retrieved on 2006-03-23.
- ? Beer T, Myrthue A (2006). "Calcitriol in the treatment of prostate cancer". Anticancer Res 26 (4A): 2647-51. PMID 16886675.
- ? Martin Mittelstaedt. "Vitamin D casts cancer prevention in new light", Global and Mail, 28 April 2007. Retrieved on 2007-04-28.
- ? Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. (2007). "Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial.". Am J Clin Nutr. 85 (6): 1586-91. PMID 17556697.
- ? Tuohimaaa and others. "Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: Vitamin D as a possible explanation.". European Journal of Cancer. PMID 17540555.
- ? "Canadian Cancer Society Recommends Vitamin D", CTV News, 8 June 2007. Retrieved on 2007-06-09.
- ? "Vitamin D 'aids lung cancer ops'", BBC News, 22 April 2005. Retrieved on 2006-03-23.
- ? Cramm KJ, Cattaneo RA, Schremmer RD (November 2006). "An infant with tachypnea". Pediatr Emerg Care 22 (11): 728–31. doi:10.1097/01.pec.0000238746.81358.ae. PMID 17110866.
- ? Walters MR, Wicker DC, Riggle PC (January 1986). "1,25-Dihydroxyvitamin D3 receptors identified in the rat heart". J. Mol. Cell. Cardiol. 18 (1): 67–72. PMID 3005597.
- ? O'Connell TD, Simpson RU (September 1996). "Immunochemical identification of the 1,25-dihydroxyvitamin D3 receptor protein in human heart". Cell Biol. Int. 20 (9): 621–4. doi:10.1006/cbir.1996.0081. PMID 8948124.
- ? Xiang W, Kong J, Chen S, et al (January 2005). "Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems". Am. J. Physiol. Endocrinol. Metab. 288 (1): E125–32. doi:10.1152/ajpendo.00224.2004. PMID 15367398.
- ? Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU (March 2007). "Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice". J. Steroid Biochem. Mol. Biol. 103 (3-5): 416–9. doi:10.1016/j.jsbmb.2006.12.081. PMID 17275288.
- ? Bourgouin P, Lucas P, Roullet C, et al (July 1990). "Developmental changes of Ca2+, PO4, and calcitriol metabolism in spontaneously hypertensive rats". Am. J. Physiol. 259 (1 Pt 2): F104–10. PMID 2375387.
- ? Simpson RU, Hershey SH, Nibbelink KA (March 2007). "Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse". J. Steroid Biochem. Mol. Biol. 103 (3-5): 521–4. doi:10.1016/j.jsbmb.2006.12.098. PMID 17275289.
- ? Simpson RU, Thomas GA, Arnold AJ (July 1985). "Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle". J. Biol. Chem. 260 (15): 8882–91. PMID 2991224.
- ? Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU (February 2008). "Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility". Endocrinology 149 (2): 558–64. doi:10.1210/en.2007-0805. PMID 17974622.
- ? Bodyak N, Ayus JC, Achinger S, et al (October 2007). "Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals". Proc. Natl. Acad. Sci. U.S.A. 104 (43): 16810–5. doi:10.1073/pnas.0611202104. PMID 17942703.
- ? Wu J, Garami M, Cheng T, Gardner DG (April 1996). "1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes". J. Clin. Invest. 97 (7): 1577–88. doi:10.1172/JCI118582. PMID 8601621.
- ? Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R (July 2003). "Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy". N. Engl. J. Med. 349 (5): 446–56. doi:10.1056/NEJMoa022536. PMID 12890843.
- ? Wolf M, Shah A, Gutierrez O, et al (October 2007). "Vitamin D levels and early mortality among incident hemodialysis patients". Kidney Int. 72 (8): 1004–13. doi:10.1038/sj.ki.5002451. PMID 17687259.
- ? Teng M, Wolf M, Ofsthun MN, et al (April 2005). "Activated injectable vitamin D and hemodialysis survival: a historical cohort study". J. Am. Soc. Nephrol. 16 (4): 1115–25. doi:10.1681/ASN.2004070573. PMID 15728786.
- ? Lee P, Eisman JA, Center JR (April 2009). "Vitamin D deficiency in critically ill patients". N. Engl. J. Med. 360 (18): 1912–4. doi:10.1056/NEJMc0809996. PMID 19403914.
- ? Martins D, Wolf M, Pan D, et al (June 2007). "Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey". Arch. Intern. Med. 167 (11): 1159–65. doi:10.1001/archinte.167.11.1159. PMID 17563024.
- ? Ford ES, Ajani UA, McGuire LC, Liu S (May 2005). "Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults". Diabetes Care 28 (5): 1228–30. PMID 15855599.
- ? Knekt P, Laaksonen M, Mattila C, et al (September 2008). "Serum vitamin D and subsequent occurrence of type 2 diabetes". Epidemiology 19 (5): 666–71. doi:10.1097/EDE.0b013e318176b8ad. PMID 18496468.
- ? Forman JP, Giovannucci E, Holmes MD, et al (May 2007). "Plasma 25-hydroxyvitamin D levels and risk of incident hypertension". Hypertension 49 (5): 1063–9. doi:10.1161/HYPERTENSIONAHA.107.087288. PMID 17372031.
- ? Bibbins-Domingo K, Pletcher MJ, Lin F, et al (March 2009). "Racial differences in incident heart failure among young adults". N. Engl. J. Med. 360 (12): 1179–90. doi:10.1056/NEJMoa0807265. PMID 19297571.
- ? Wolf M, Betancourt J, Chang Y, et al (July 2008). "Impact of activated vitamin D and race on survival among hemodialysis patients". J. Am. Soc. Nephrol. 19 (7): 1379–88. doi:10.1681/ASN.2007091002. PMID 18400938.
- ? Zittermann A, Schleithoff SS, Koerfer R (October 2005). "Putting cardiovascular disease and vitamin D insufficiency into perspective". Br. J. Nutr. 94 (4): 483–92. PMID 16197570.
- ? Holick MF (July 2007). "Vitamin D deficiency". N. Engl. J. Med. 357 (3): 266–81. doi:10.1056/NEJMra070553. PMID 17634462.
- ? Boulay F, Berthier F, Sisteron O, Gendreike Y, Gibelin P (July 1999). "Seasonal variation in chronic heart failure hospitalizations and mortality in France". Circulation 100 (3): 280–6. PMID 10411853.
- ? Poole KE, Loveridge N, Barker PJ, et al (January 2006). "Reduced vitamin D in acute stroke". Stroke 37 (1): 243–5. doi:10.1161/01.STR.0000195184.24297.c1. PMID 16322500.
- ? Dobnig H, Pilz S, Scharnagl H, et al (June 2008). "Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality". Arch. Intern. Med. 168 (12): 1340–9. doi:10.1001/archinte.168.12.1340. PMID 18574092.
- ? Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. (1990). "Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study.". Int J Epidemiol. 19 (3): 559-563. PMID 2262248.
- ? Grimes DS, Hindle E, Dyer T. (1996). "Sunlight cholesterol and coronary heart disease.". Quarterly Journal of Medicine 89 (8): 579-589. PMID 8935479.
- ? Spencer FA, Goldberg RJ, Becker RC, Gore JM. (1998). "Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction.". J Am Coll Cardiol. 31 (6): 1226-33. PMID 9581712.
- ? Ku CS, Yang CY, Lee WJ, Chiang HT, Liu CP, Lin SL. (1998). "Absence of a seasonal variation in myocardial infarction onset in a region without temperature extremes.". Cardiology. 89 (4): 277-82. PMID 9643275.
- ? Giovannucci E, Liu Y, Hollis BW, Rimm EB (June 2008). "25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study". Arch. Intern. Med. 168 (11): 1174–80. doi:10.1001/archinte.168.11.1174. PMID 18541825.
- ? Melamed ML, Muntner P, Michos ED, et al (June 2008). "Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004". Arterioscler. Thromb. Vasc. Biol. 28 (6): 1179–85. doi:10.1161/ATVBAHA.108.165886. PMID 18417640.
- ? Reis JP, Michos ED, von Mühlen D, Miller ER (December 2008). "Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease". Am. J. Clin. Nutr. 88 (6): 1469–77. doi:10.3945/ajcn.2008.26447. PMID 19064505.
- ? Bodyak N, Ayus JC, Achinger S, et al (October 2007). "Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals". Proc. Natl. Acad. Sci. U.S.A. 104 (43): 16810–5. doi:10.1073/pnas.0611202104. PMID 17942703.
- ? Park CW, Oh YS, Shin YS, et al (January 1999). "Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism". Am. J. Kidney Dis. 33 (1): 73–81. PMID 9915270.